How Many Moles of Water Are Present in 75.0 G
Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. 3NO2H2O 2HNO3NO Chemistry HELP In a constant-pressure calorimeter 500 mL of 0300 M Ba OH2 was added to 500 mL of 0600 M HCl.

Solved 1 How Many Moles Of Water H20 Are Present In 75 0 Chegg Com
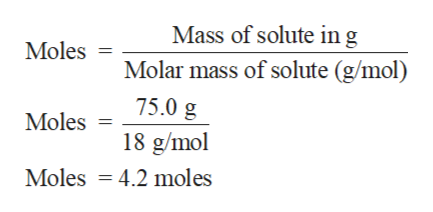
Mass of H2O 750 g Molar mass of H2O 18 gmol.
. Water has a molar mass of 18015 gmol. Density of water 1 gml Medium. How many moles of water H2O are present in 750 g H2O.
Water has a molar mass of 180153 grams per mole. 5988 kg 5988 g. A T chart is useful for this kind of problem.
Which means that it will contain. How many molecules of water H2O are present in 750 g of H2O. Imagine you have 6 liters of pure water.
Chemistry questions and answers. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O. How many moles of water H20 are present in 750 g of H20.
View the full answer Transcribed image text. THIS IS THE BEST ANSWER 417 moles. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg.
The molecular mass of water is calculated as follows. 135 x 103 moles 417 moles 441 moles 750 moles 750 moles FLAGjpg 1s. Mass of the water given 75 g.
So to sum this up 60221023 molecules of water will amount to 1 mole of water which in turn will have a mass of 18015 g. Avogadros number is the number of. The number of atoms present in 4167 moles of water is calculated as follows.
How many moles of water H2O are present in 750 g H2O. If 1 0 4 d m 3 of water is introduced into a 10 d m 3 flask at 300 K then how many moles of water are in the vapour phase when equilibrium is. The molecular formula for Water is H2O.
1 mole of H2O Contain 6023 x 1023. Calculate the molar mass of potassium chloride KCl. 1 grams Water is equal to 0055508435061792 mole.
Which statement best explains the symbol nacl. The SI base unit for amount of substance is the mole. 750 g H2O x 1 mole1799 g x 602x1023 moleculesmole 251x1024 molecules.
Number of moles of water in 18 litre of pure water is. The number of moles of 75 g of water is calculated as follows. Its easier to work with grams so convert the mass.
A 441 mol B 416 mol a 750 mol D 750 mol El 135 x 10 mol Ο Α B Ос OD Question 18 1 pts A sample of a white solid contains 0212 g of magnesium and 0140 g oxygen. How many moles of water are present in one litre of water. This means that one mole of water molecules has a mass of 18015 g.
1 mole molar mass. Number of moles mass in gram Molar mass 750 g 18 gmol 417 mol. 44813 results page 30.
0795L solution 0870 moles KBr 1L solution 069165 moles KBr. How many moles of water H2O are present in 750 g HO. Now potassium bromide has a molar mass of 119002 g mol1 which means that every mole of potassium bromide has a mass of 119002 g.
Moles of How many moles of oxygen o2 are present in 336 l of the How many moles of water h2o contain 201022 molecules of. The answer is 1801528. H₂O 2x 1 16 18 gmol.
From there you can go from moles to molecules using 6022 1023 molecules in a mole. 44784 results Chemistry How Many Moles Of H2O Are Required To Produce 45 Moles Of HNO3 According To The Following Reaction. How many atoms of chlorine are in 100 mol of chlorine gas.
The number of hydrogen atoms present in 75 grams of water is 502 x 10²⁴ atoms. 75 grams of the substance is then equal to 416 moles of water. 1801528 grams of water per mole of water.
You can view more details on each measurement unit. In your case the solution is said to have a volume of. Particles in 1 mol of a substance.
135 103 moles e. Find the molar mass of a water molecule H₂O. How many moles of water h2o are present in 750 g h2o.
795mL 1 L 103mL 0795 L. How many molecules of water H2O are present in 750 g of H2O. One mol of potassium weighs _____ 3910 g.
120 10 24. 1 mole 6023 x 1023 molecules. Helpful 0Not Helpful 0 Add a.
502 x 1024 molecules 753 x 1024 molecules 417 molecules 251 x 1024 molecules 750 molecules Question 4 1 pts How many moles of water H20 are present in 750 g of H2O. We assume you are converting between grams Water and mole. 27144moles H2O 60221023molec1mole H2O 16351024molec.
How many moles of water H2O are present in 750 g H2O. Molecular weight of Water or mol. How many moles of water H2O are present in 750 g H2O.
O 417 moles O 441 moles O 135 x 103 moles O 750 moles O 750 moles. The molar mass of water is 18 g mol Now given 750 g of water sampl.
Solved How Many Molecules Of Water H2o Are Present In 75 0 Chegg Com

Solved How Many Moles Of Water H20 Are Present In 75 0 G Chegg Com

Comments
Post a Comment